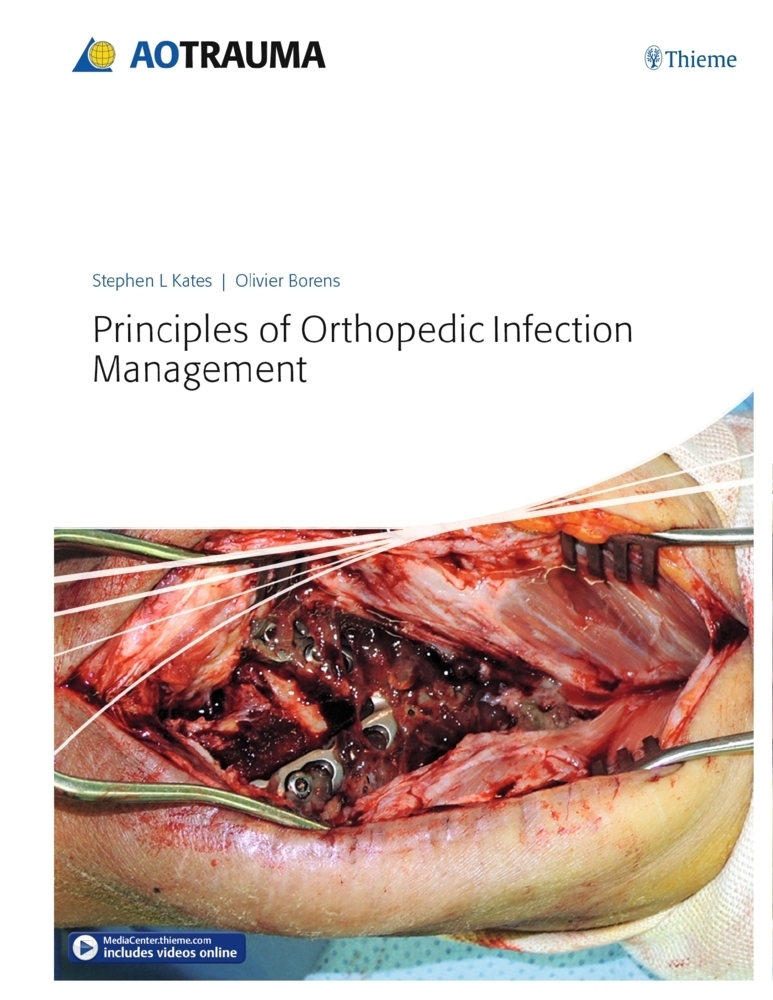
56,35 €*
Versandkostenfrei per Post / DHL
Lieferzeit 1-2 Wochen
Vaccination programmes are of vital importance to public health and are present in virtually every country in the world. By promoting an understanding of the diverse effects of vaccination programmes, this textbook discusses how epidemiologic methods can be used to study, in real life, their impacts, benefits and risks.
Vaccination programmes are of vital importance to public health and are present in virtually every country in the world. By promoting an understanding of the diverse effects of vaccination programmes, this textbook discusses how epidemiologic methods can be used to study, in real life, their impacts, benefits and risks.
Susan Hahné is a senior epidemiologist in the National Immunisation Programme Department at the Centre for Epidemiology and Surveillance of Infectious Diseases of the National Institute for Public Health and the Environment (RIVM) in the Netherlands.
Kaatje Bollaerts is the head of data science at P95 Belgium, a scientific service-providing company focused on pharmacovigilance and epidemiology related to vaccines and infectious diseases.
Paddy Farrington is an emeritus professor of statistics at the Open University (OU), UK. Prior to joining the OU in 1998, he was a statistician at the Immunisation Division of the Communicable Disease Surveillance Centre in the UK for 11 years
Part I: Background 1.Vaccines 2.How vaccines work 3.Vaccination programmes 4.Dynamics of controlling vaccine preventable diseases 5.Impact of mass vaccination programmes 6.Vaccine risks: a societal perspective Part II: Field Tools for Monitoring Vaccination Programmes 7.Monitoring vaccine coverage and attitudes towards vaccination 8.Surveillance of vaccine preventable diseases and pathogens 9.Serological surveillance 10.Assessing impact 11.Outbreak investigation of vaccine preventable diseases Part III: Vaccine Effectiveness 12.Vaccine effectiveness 13.Estimating vaccine effectiveness: General methodological principles 14.Estimating vaccine effectiveness: Cohort and household contact studies 15.Estimating vaccine effectiveness: Case-control and screening studies 16.Waning vaccine effectiveness and models of vaccine action Part IV: Risks Associated with Vaccination Programmes 17.Vaccine safety: an introduction 18.Surveillance of adverse events following immunisation 19.Estimating vaccination risks: general methodological principles 20.Epidemiological study designs for evaluating vaccine safety Part V: Benefit-Risk Assessment of Vaccination Programmes 21.Benefit-risk assessment of vaccination programmes
| Erscheinungsjahr: | 2021 |
|---|---|
| Produktart: | Ratgeber |
| Rubrik: | Sachliteratur |
| Medium: | Taschenbuch |
| Seiten: | 446 |
| Inhalt: | Einband - flex.(Paperback) |
| ISBN-13: | 9781138054851 |
| ISBN-10: | 1138054852 |
| Sprache: | Englisch |
| Einband: | Kartoniert / Broschiert |
| Autor: |
Bollaerts, Kaatje
Farrington, Paddy Hahne, Susan |
| Hersteller: | Taylor & Francis Ltd |
| Maße: | 175 x 246 x 35 mm |
| Von/Mit: | Kaatje Bollaerts (u. a.) |
| Erscheinungsdatum: | 14.09.2021 |
| Gewicht: | 0,828 kg |
Susan Hahné is a senior epidemiologist in the National Immunisation Programme Department at the Centre for Epidemiology and Surveillance of Infectious Diseases of the National Institute for Public Health and the Environment (RIVM) in the Netherlands.
Kaatje Bollaerts is the head of data science at P95 Belgium, a scientific service-providing company focused on pharmacovigilance and epidemiology related to vaccines and infectious diseases.
Paddy Farrington is an emeritus professor of statistics at the Open University (OU), UK. Prior to joining the OU in 1998, he was a statistician at the Immunisation Division of the Communicable Disease Surveillance Centre in the UK for 11 years
Part I: Background 1.Vaccines 2.How vaccines work 3.Vaccination programmes 4.Dynamics of controlling vaccine preventable diseases 5.Impact of mass vaccination programmes 6.Vaccine risks: a societal perspective Part II: Field Tools for Monitoring Vaccination Programmes 7.Monitoring vaccine coverage and attitudes towards vaccination 8.Surveillance of vaccine preventable diseases and pathogens 9.Serological surveillance 10.Assessing impact 11.Outbreak investigation of vaccine preventable diseases Part III: Vaccine Effectiveness 12.Vaccine effectiveness 13.Estimating vaccine effectiveness: General methodological principles 14.Estimating vaccine effectiveness: Cohort and household contact studies 15.Estimating vaccine effectiveness: Case-control and screening studies 16.Waning vaccine effectiveness and models of vaccine action Part IV: Risks Associated with Vaccination Programmes 17.Vaccine safety: an introduction 18.Surveillance of adverse events following immunisation 19.Estimating vaccination risks: general methodological principles 20.Epidemiological study designs for evaluating vaccine safety Part V: Benefit-Risk Assessment of Vaccination Programmes 21.Benefit-risk assessment of vaccination programmes
| Erscheinungsjahr: | 2021 |
|---|---|
| Produktart: | Ratgeber |
| Rubrik: | Sachliteratur |
| Medium: | Taschenbuch |
| Seiten: | 446 |
| Inhalt: | Einband - flex.(Paperback) |
| ISBN-13: | 9781138054851 |
| ISBN-10: | 1138054852 |
| Sprache: | Englisch |
| Einband: | Kartoniert / Broschiert |
| Autor: |
Bollaerts, Kaatje
Farrington, Paddy Hahne, Susan |
| Hersteller: | Taylor & Francis Ltd |
| Maße: | 175 x 246 x 35 mm |
| Von/Mit: | Kaatje Bollaerts (u. a.) |
| Erscheinungsdatum: | 14.09.2021 |
| Gewicht: | 0,828 kg |